
 Dr. KK Aggarwal
Dr. KK Aggarwal
Everything you need to know about Russian Sputnik V Vaccine
Russian Sputnik v Vaccine Update
More than 200 different COVID-19 vaccines are currently under production around the world.
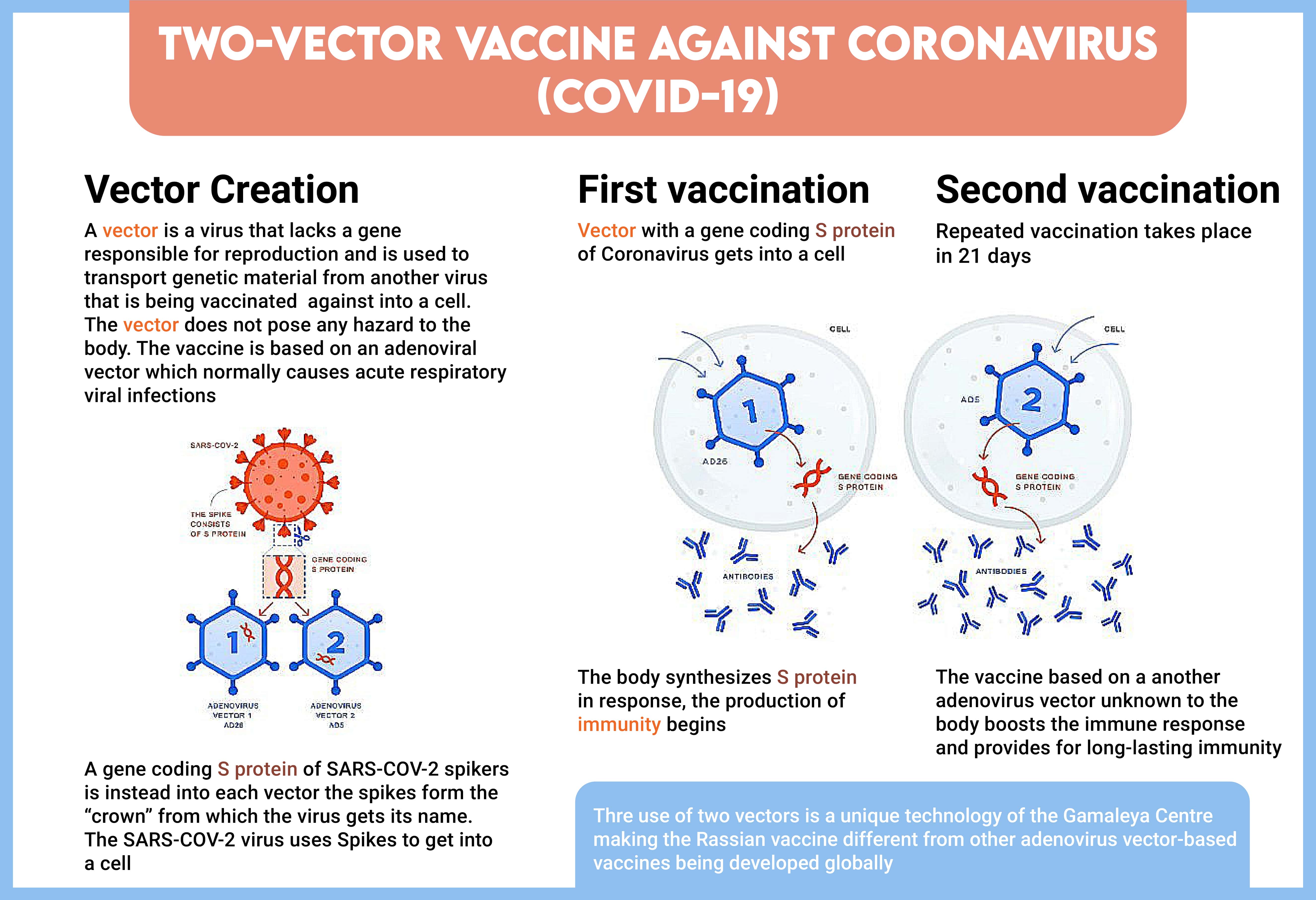
Sputnik V is the world’s first registered vaccine based on a well-studied human adenoviral vector-based platform. It currently ranks on the World Health Organization (WHO) list among the top-10 candidate vaccines approaching the end of clinical trials and the start of mass production.
40,000 participants are interested in the ongoing Sputnik V post-registration clinical trial in Russia.
In the UAE, India, Venezuela and Belarus, clinical trials have been announced for Sputnik V.
How Sputnik V Is Engineered To Work
Clinical Trials
The vaccine went through all stages of pre-clinical trials with studies on various species of animals, including 2 types of primates, prior to the start of clinical trials.
On August 1 , 2020, the Phase 1 and 2 clinical trials of the vaccine were completed. All the volunteers feel well and no unexpected or unpleasant side effects have been observed.
A strong antibody and cellular immune response were induced by the vaccine. Since administering the vaccine, not a single participant in the latest clinical trials was infected with COVID-19.
The high efficacy of the vaccine was verified by high-precision tests for volunteer blood serum antibodies (including examination for coronavirus-neutralizing antibodies) as well as the ability of volunteer immune cells to activate in response to the coronavirus spike S protein, suggesting the development of both antibody and cellular immune vaccine responses.
On August 25, 2020, post-registration clinical trials were initiated affecting more than 40,000 people in Russia and Belarus.
A number of countries, including the UAE, India, Venezuela, Egypt and Brazil, will participate locally in the Sputnik V clinical trials.
The vaccine obtained a certificate of registration from the Russian Ministry of Health on August 11 and could be used to vaccinate the population in Russia under emergency rules introduced during the COVID-19 pandemic.
The aim is to scale up the production of vaccines in Russia and globally.
Gamaleya's National Research Institute of Epidemiology and Microbiology has secured patent rights in Russia for the special substance and method of use of Sputnik V.
Read more - Should I Get Tested For Coronavirus?
Good News For India
If all goes well, Phase II clinical trials of the Russian Sputnik V vaccine will begin by the end of this month at the West Bengal State Government-run College of Medicine and Sagore Dutta Hospital, a senior health department official said on Tuesday.
A site management organisation performed the requisite surveys, including visits to the hospital to review its infrastructure and cold storage facilities, before starting the process, he said.
Snehendu Koner, head of business development at the CliniMed LifeSciences site management organisation, said a report on the results of the survey was submitted for approval to the Drug Control General of India (DCGI).
"We visited the site and carried out inspections of its infrastructure, as well as vaccine storage facilities and immunogenicity samples."
"We have also gone through the records of the hospital and discovered that it has clinical trial experience. Our results are very satisfactory and we have submitted it for approval to the DCGI," Koner told PTI when contacted.
Once the DCGI generates a green signal, the Ethics Committee of the hospital will issue a clearance to begin clinical Phase II trials there, he said.
"We have named the lead investigator for the procedure as well as the co-investigator," he said.
The pharmaceutical company Dr Reddy 's Lab, which has joined hands with the Russian Direct Investment Fund (RDIF), will conduct the clinical trial of Sputnik V, scheduled to take place in the world.
The RDIF will supply 100 million doses of its possible COVID-19 vaccine to Dr Reddy 's Lab, according to a health department official.
One hundred volunteers will be selected nationally, and 75 of them will receive the vaccine. Placebo, a drug or medication that is supposed to have no medicinal benefit, will be offered to twenty five others.
Read more

Dr. KK Aggarwal
Recipient of Padma Shri, Vishwa Hindi Samman, National Science Communication Award and Dr B C Roy National Award, Dr Aggarwal is a physician, cardiologist, spiritual writer and motivational speaker. He was the Past President of the Indian Medical Association and President of Heart Care Foundation of India. He was also the Editor in Chief of the IJCP Group, Medtalks and eMediNexus















Please login to comment on this article